A Company Benchmark of Packaged First Foods in Southeast Asia
COMMIT is the Consortium for Improving Complementary Foods in Southeast Asia. It was established to better understand the use, quality and regulation of commercially produced complementary foods (CPCF) in seven Southeast Asian countries: Cambodia, Indonesia, the Lao People’s Democratic Republic (PDR), Malaysia, the Philippines, Thailand and Viet Nam.
ATNI’s role in COMMIT is to report on how food and beverage companies’ CPCF products perform against an adapted version of the nutrient profile model for CPCF developed by the WHO Regional Office for Europe (referred to hereinafter as the adapted WHO Europe NPM for CPCF). Scroll down to check the company findings and click on the map to access the full country reports.
COMMIT partners include ATNI; Alive & Thrive; Helen Keller International; JB Consultancy; School of Food Science, University of Leeds; UNICEF East Asia and the Pacific Regional Office; and World Food Programme Asia Pacific Regional Office.
Click here to learn more about all research activities conducted by COMMIT and explore the available resources.
Complementary feeding in Southeast Asia and the market for Commercially Produced Complementary Foods
The complementary feeding period, between six months and three years of age, is a critical phase during which foods of inadequate quality and quantity can have a long-term impact on children’s growth and development.
As millions of families in Southeast Asia shift from providing older infants and young children with traditional meals towards processed, convenience store-bought foods, it is essential that they are provided with nutritious, safe, and diverse foods that are affordable and introduced at the right time. This is particularly crucial as malnutrition in all its forms is a persistent concern in Southeast Asia.
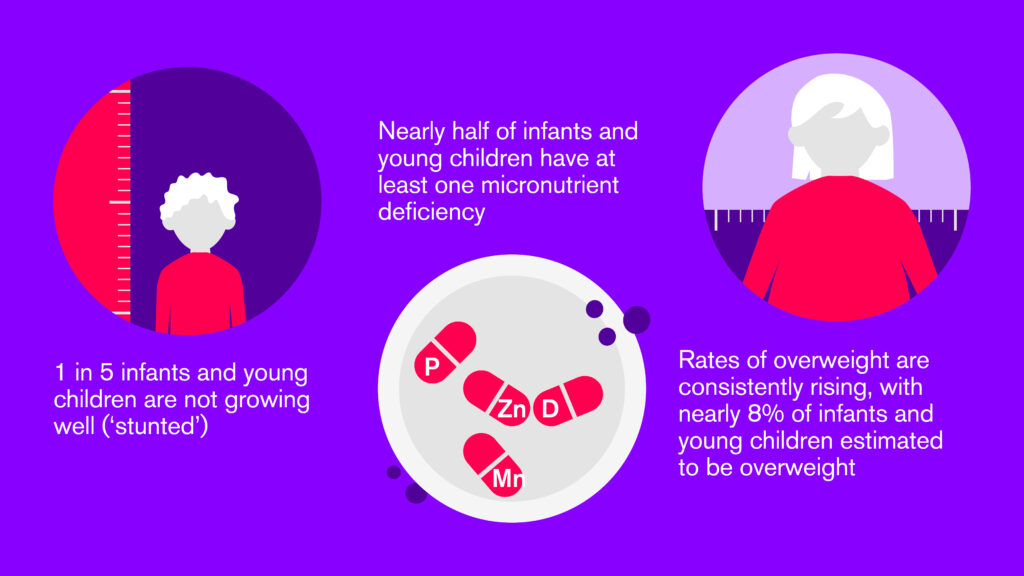
Commercially Produced Complementary Foods (CPCF) can vary widely in nutritional quality. Although fortified CPCFs have the potential to improve the intake of critical micronutrients that may be limited in infant and young children’s diets, other CPCFs are high in added sugar and salt and contain unhealthy fats.
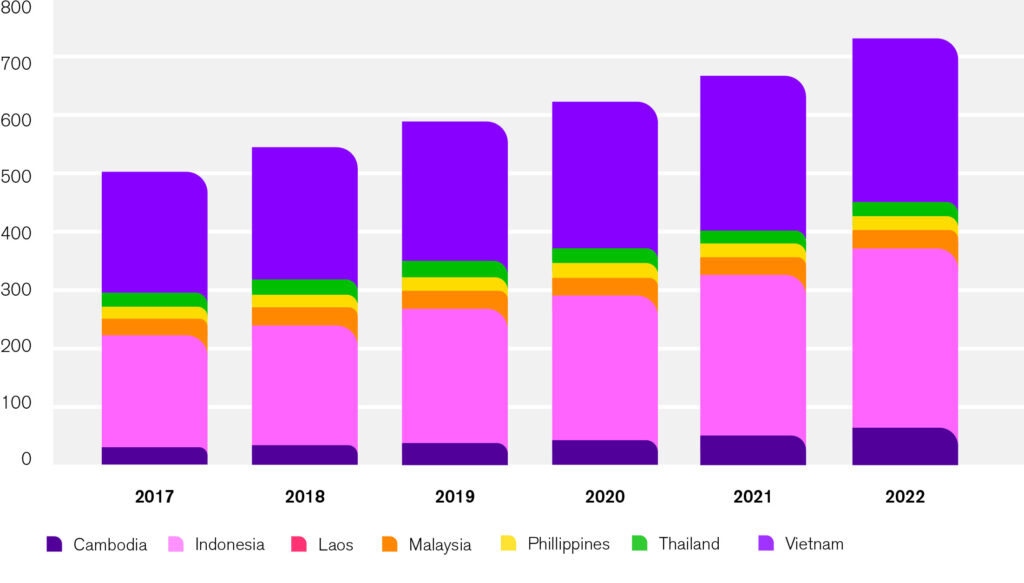
CPCF sales have been on the rise in the Southeast Asia region. Over the past five years, the CPCF market in seven countries of the region grew by almost 45%, amounting to over USD 750 million in aggregated retail sales in 2022. Among the seven countries, Indonesia has the largest CPCF market, closely followed by Vietnam.
[Source: Euromonitor International 2017-2022]
Aggregate CPCF sales (in USD million)
The role of food and beverage companies
A concern identified by the United Nations Children Fund East Asia and the Pacific Regional Office (UNICEF EAPRO) is that processed foods higher in salt, sugar, and unhealthy fats, and lower in essential nutrients, are increasingly being marketed and consumed in the region. Therefore, at the food systems level, key points of action are incentivizing the production of nutritious and safe CPCF and regulating the marketing and labeling of unhealthy foods and beverages to children.
Food and beverage companies selling CPCF products are at the forefront of creating an enabling food environment that can improve the status of complementary feeding, and thus the nutrition and health of older infants and young children in Southeast Asia.
Company performance on the assessments against the adapted WHO Europe NPM
One of COMMIT’s workstreams involves an assessment using an adapted version of the nutrient profile model for CPCF developed by the WHO Regional Office for Europe (referred to hereinafter as the adapted WHO Europe NPM for CPCF). This model was used to profile the nutrient composition and labeling practices of CPCF sold in the capital cities of and through online retailers in the seven Southeast Asian countries.
As one of COMMIT’s partners, ATNI’s role is to report on the performance of companies whose products were assessed against the nutritional and labeling requirements of the adapted NPM for CPCF.
Click here for more details about the adapted WHO Europe NPM for CPCF. More information can also be found on the COMMIT website.
CPCF nutritional composition
- CPCF are categorized across 5 food categories and 16 subcategories. The ingredient list and nutritional content for each unique CPCF were cross-checked against subcategory-specific nutrient/ingredient requirements in the adapted NPM for CPCF.
- A product ‘passed’ the CPCF NPM nutrient composition assessment if it met all category-specific nutrient requirements (as applicable).
- If a product ‘failed’ any of the applicable category-specific nutrient composition requirements, it ‘failed’ the full nutrient composition assessment.
- In cases where product labels were missing nutrient content information, the products ‘failed’ the thresholds.
CPCF labeling practices
- A product ‘passed’ the CPCF NPM labeling practices assessment if it met all applicable labeling requirements.
- If a product ‘failed’ any of the applicable labeling requirements, it ‘failed’ the full assessment on labeling practices.
- If a product was missing information for one of the applicable labeling practices components, for example product name and ingredient list clarity, it ‘failed’ the labeling requirement.
Combined assessments of CPCF nutrient composition and labelling practices
- A CPCF must meet all category-specific nutrient composition and labeling requirements combined to be considered suitable to be promoted for older infants and young children between six months and three years of age.
Regional key findings
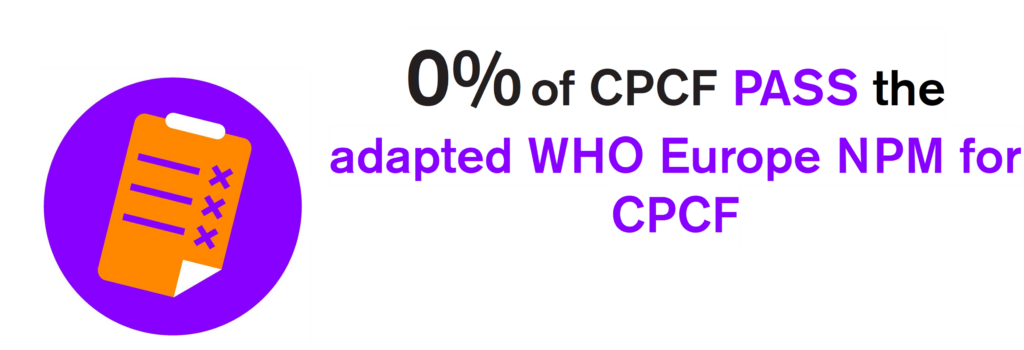
Results of the combined assessment of CPCF nutrient composition and labeling practices
None of the companies’ CPCF products ‘passed’ all requirements of the adapted NPM for CPCF.
Results on nutritional composition requirements
As shown below, all products from 15 out of the 113 companies passed all nutrient composition requirements. However, a large number of companies (n=42) had none of their products pass all nutrient composition requirements. The remaining companies had at least one product meet such requirements. Click under each of the ranges below to see the companies which had CPCF passing nutrition requirements within each range.
Companies seem to be meeting the fat requirement well, as around 95% of products met this requirement. Overall, companies had almost 63% of their CPCF meet the sodium thresholds; a lower percentage was observed for having ‘no added sugar/ sweeteners’ (~56%).
Companies had 100% of their products* passing all nutrient composition requirements
Companies had >75%-<100% of their products* passing all nutrient composition requirements
Companies had >50%-75% of their products* passing all nutrient composition requirements
Companies had >25%-50% of their products* passing all nutrient composition requirements
Companies had > 0-25% of their products* passing all nutrient composition requirements
Companies had 0% of their products* passing all nutrient composition requirements
Results on labeling requirements
None of the companies passed all labeling requirements for specific criteria. Companies performed worst in meeting all labeling requirements on claims (almost 1% of CPCF products met all claims requirements), followed by labeling requirements on breastfeeding (around 17% of CPCF products met all breastfeeding requirements). In comparison, companies performed better in meeting all labeling requirements on ingredients (46.5%).
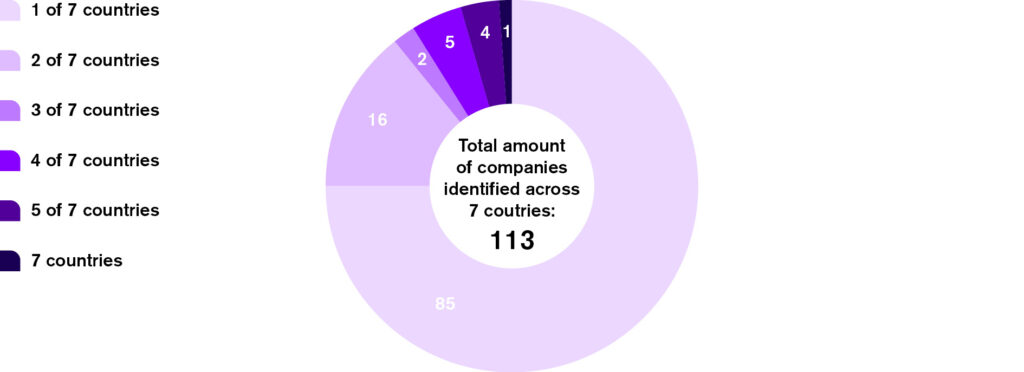
Variation in company performance across countries
Of the 113 companies assessed, 28 had CPCF identified in more than one country. In many cases, the findings of a company’s CPCF products varied between countries. Differences were especially notable for certain criteria of the adapted WHO Europe NPM for CPCF. This occurs for several reasons, including differences in the CPCF nutrient content and labeling standards in each country, but also the types of CPCF marketed in each country.
Danone’s assessment exemplifies how a company’s performance can vary depending on the product types available in a market. In Vietnam, all of Danone’s identified CPCF met the nutrient content requirements – these products were categorized as ‘dry or instant cereals/starch’. In Lao PDR, however, none of Danone’s identified CPCF ‘passed’ the nutritional assessment. Among the four CPCF identified in Lao PDR, three were directly excluded from the assessment as they were categorized as ‘confectionery, sweet spreads, and fruit chews’; products that should not be promoted to children under three years of age. The remaining product assessed against the adapted NPM was a ‘snack/finger food’.
Key recommendations for companies selling CPCF
- All companies are urged to consider both the nutrient composition and appropriate labeling of their CPCF products to ensure they are suitable to promote for older infants and young children under three years of age.
- Companies are encouraged to continuously improve their CPCF products in line with relevant developments in national, regional, and global guidance. Check the COMMIT website for further resources.
- Companies should refrain from producing and selling to children under three years of age any confectionery, sweet spreads, fruit chews, and juices, as well as other drinks, including milks that have added sugars and sweeteners.
- Companies are also urged to reduce the sugar and sodium content of their CPCF and eliminate the use of added sugars and sweeteners in these products.
- Products of the same food category from different companies have shown varying results in nutrient composition. These range from products that do not meet any nutritional requirements to those that fully ‘pass’ the nutritional assessment – and this must serve as a demonstration to companies that it is possible to improve the nutritional composition of their CPCF product portfolio.
- No company fully ‘passed’ the labeling criteria. Thus, companies are urged to improve the labeling of their CPCF to ensure messaging that protects exclusive and continued breastfeeding, and instructs on age-appropriate and safe use of blended/puréed CPCF, including spouted products. CPCF labels must also have complete information about the products’ ingredient lists but should not carry any form of claims (except for permitted compositional claims).
- The research has shown that, among a company’s CPCF products, some fully meet certain labeling requirements, while others do not meet any – even where these products were found in the same country. ATNI calls on companies to ensure consistent best practices among all products, in all the markets where their CPCF are sold.
- Another notable finding was that several product labels in Cambodia, Indonesia, Lao PDR, the Philippines, and Viet Nam were neither in English nor in the local language. Companies must ensure controlled sales of their products to only reach the intended markets, and that they are available in the local language of the consumers to ensure awareness of the product and its appropriate use.