Action Research on Commercially Produced Complementary Foods
The Access to Nutrition Initiative’s (ATNi) goal is to drive private sector action to address the nutrition challenges faced by all countries. ATNi aims to achieve this goal by undertaking research and developing tools and initiatives to track and drive a greater contribution by the food and beverage sector to improving diets, particularly those of infants and young children.
In 2020, ATNi commissioned the Nutrition Center of the Philippines (NCP) to undertake two separate but linked pieces of research and assessment, on a pilot basis, to answer two key questions in respect older infants and young children in the Philippines:
- Landscape Analysis: Infant and Young Child Feeding: What is the status of older infants’ and young children’s nutrition, diets and health, what influences it and how can it be improved?
- Product Profile of Commercially Produced Complementary Foods (CPCF): More specifically, do these products meet the nutritional standards of the WHO Europe’s newly developed nutrient profiling model (WHO NPM) designed to assess the nutritional quality of CPCF marketed as suitable for older infants and young children (6-36 months), and whether are they labelled in line with WHO recommendations.
The ultimate goal of this research is to improve the nutritional quality of CPCF in the Philippines and to ensure that these foods are appropriately labelled.
Full reportThe landscape analysis collates the most up-to-date statistics on the status of older infants and young children’s diets in the Philippines. It found that Filipino children suffer from all forms of malnutrition. More than 20% of children are stunted and or have various micronutrient deficiencies; some 7% are wasted and 3% are overweight, conditions all due to inadequate nutrition in the first years of life.
Exclusive breastfeeding rates in the first six months remain low at only 35% in 2019, although mark an improvement from the rate of 24.5% in 2015. The proportion of children 6- to 23-months-old that have a minimum acceptable diet, i.e., which met both minimum dietary diversity and minimum meal frequency to ensure both dietary quality and nutrient adequacy significantly decreased between 2015 and 2019 from 18.6% to 9.9%.
Recent studies conducted by the Philippines’ Food and Nutrition Research Institute have shown that carbohydrate food sources dominate the food intake of older infants and young children at an age when their development requires more energy and macronutrients. These studies also found that the majority of children have poor protein and fat intake.
In addition, more than 50% of children have an inadequate dietary intake of iron, Vitamin A, calcium, Vitamin C and other B vitamins, and less than 10% consumed fruits or vegetables. Just over half (52%) of children aged 6-23 months reportedly consume commercial baby food, but there is no publicly available data as to what proportion of their diets these products comprise.

The second part of the research was an assessment of the nutritional composition and labelling of CPCF found in the National Capital Region of the Philippines using the WHO Europe NPM designed specifically to assess CPCF marketed as suitable for older infants and young children (6-36 months). Although ATNi recognizes that this model was developed for the Europe region, it was used for this pilot study as there is not yet an equivalent model adapted for the south-east Asia region, or for the Philippines.
A total of 211 CPCF marketed as suitable for older infants and young children between 6 and 36 months of age were found and assessed. These products were made by 22 food companies, 19 of which are based outside of the Philippines.
Almost all (98%) of the products were manufactured outside of the country, imported and distributed either by official distributors or by local sellers, typically through online selling platforms and large grocery retailers. Out of all the products, only seven of the nine made by Filipino companies carried the Philippine FDA Registration number on pack.
Nutritional Composition
The WHO Europe NPM pays particular attention to the marketing of CPCFs high in fat, sugars and salt. Nutritional thresholds against these three key nutrients apply to all types of CACFs. The NPM also assesses against nutritional thresholds that are specific to the different categories of CPCFs such as soft-wet spoonable foods/ready-to-eat foods, dry and instant cereal or starch, meals with chunky pieces, and snacks and finger foods.
In terms of the proportion of the 211 products that met the three key nutrition criteria, there were some positive results:
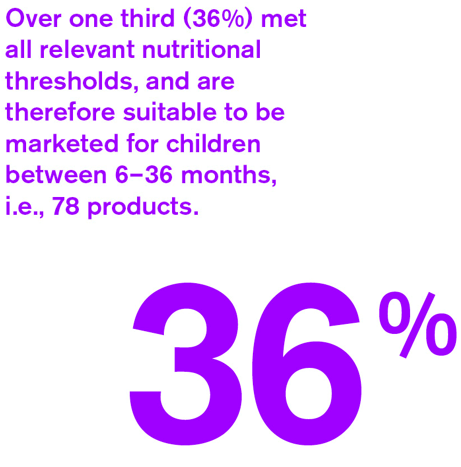
- Over one third (36%) met all relevant nutritional thresholds, and are therefore suitable to be marketed for children between 6–36 months, i.e., 78 products.
- More than two-thirds (69%) of the products did not have any added sugars or sweeteners.
- Just over three-quarters (78%) had the recommended sodium content.
- Almost all (98%) of the products met the recommended fat content based on current European Council regulations.
The proportion of products within each CPCF category that met ALL relevant nutritional thresholds were as follows:
- 54% of dry and instant cereal/starchy foods
- 43% of the soft-wet spoonable, ready-to-eat foods
- 11% of the snacks and finger foods
- one of the two meals with chunky pieces
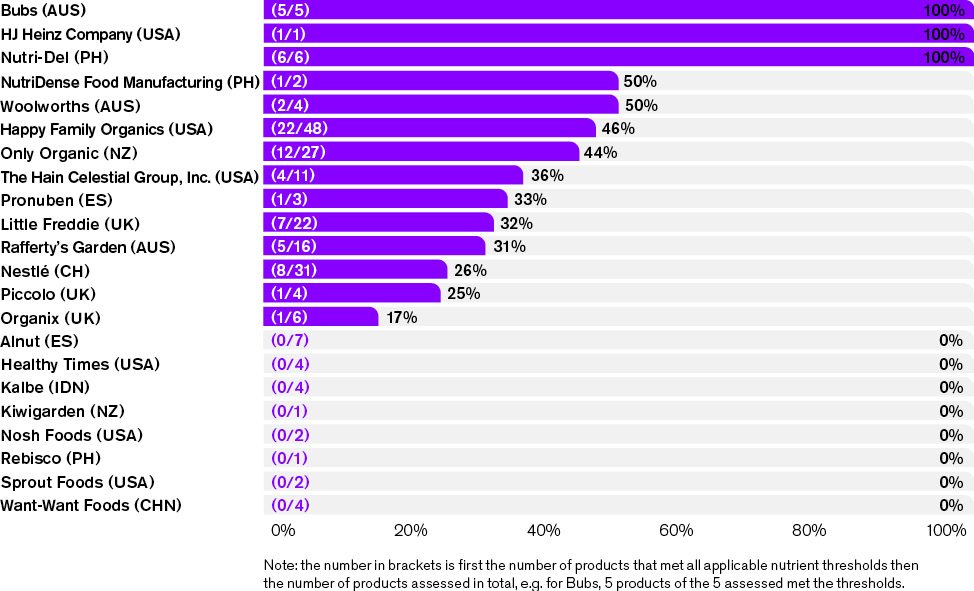
These figures demonstrate that some companies are making CPCF of the appropriate nutritional quality for older infants and young children. The companies were ranked according to the percentage of their products that met all applicable nutrient thresholds.
The pattern most evident is that those companies that perform poorly (with no products meeting all criteria) mainly do so because their products had added sugar/sweeteners and inappropriately high sodium levels.
Labelling
Marketing messages and product labels were assessed against WHA 69.9 recommendations and the additional criteria set out by WHO Europe. Some findings were positive. A large majority (89%) of products’ packaging did not include health claims. Similarly, the vast majority of products were labelled in accordance with the recommendations of WHA 69.9, in that complementary foods should not be introduced earlier than six months of age. Many of the other findings were less encouraging. The total sugar content of well over half of the products assessed exceeded the level for which WHO Europe proposes a FOP warning label be used to show the percentage of energy from total sugar. More than four fifths (84%) made nutrition and /or composition claims and only 5% included a message about the importance of continued breastfeeding alongside complementary feeding, up to two years of age or beyond. Overall, none of the product labels by any company fully adhered to all WHA and WHO recommendations.
While some companies already make products of appropriate nutritional quality to be marketed as suitable for older infants and young children, the proportion of such products on the Philippine market is low. Considering that older infants and young children are a highly vulnerable group and the importance of establishing healthy eating habits (which confer benefits throughout life), early, it is critical that CPCF have both an appropriate composition and are appropriately labelled. All manufacturers of CPCF have the opportunity to make a positive contribution to improving the growth and development of older infants and young children in the Philippines – and elsewhere – by improving the formulation of their products. Moreover, the importance of labelling CPCF appropriately, according to current standards and the recommendations of the WHO, is also clearly apparent.
To set nutritional standards for CPCF manufacturers to follow, there is clear value in the WHO developing NPMs for these products adapted to each region as needed, with associated recommendations in relation to the labelling and marketing of these products adapted as necessary to each region’s specific context. Such regional models could then be used by countries in the region, or countries could develop their own.
To set nutritional standards for CPCF manufacturers to follow, there is clear value in the WHO developing NPMs for these products adapted to each region as needed, with associated recommendations in relation to the labelling and marketing of these products adapted as necessary to each region’s specific context. Such regional models could then be used by countries in the region, or countries could develop their own.
All manufacturers of CPCF have the opportunity to make a positive contribution to improving the growth and development of older infants and young children in the Philippines – and elsewhere – by improving the formulation of their products. Moreover, the importance of labelling CPCF appropriately, according to current standards and the recommendations of the WHO, is also clearly apparent.
Full report