
Complementary Foods Product Profile 2024
Introduction
For infants and young children, the WHO recommends introducing safe and nutrient-rich foods when breast milk or milk formula alone are no longer meet nutritional requirements of growing infants. This period, known as complementary feeding, starts at the age of 6 months and continues up to 2 years of age or beyond, with continued breastfeeding. It is a critical period that can influence the short and long-term health outcomes of infants and young children.
Over the last decade, there has been substantial global growth in the CACF market, retail sales increased by around 20% from 2019 to 2023. The growth differs across regions as almost 30% volume growth has been in Asia and Pacific regions and 10% in European region in the past 5 years.
In 2020, ATNi used the draft version of WHO Europe’s nutrient profiling model for CACF in conducting an assessment of CACF in the Philippines. Following this research, ATNi along with its partners of COMMIT used a version of WHO Europe’s draft model to assess CACF in seven Southeast Asian countries.
For the present study, ATNi used the 2022 WHO Nutrient and Promotion Profile Model (NPPM).
The primary objective of this research is to increase the body of evidence on the nutritional quality and labelling practices of CACF, ensuring that more countries across different regions are covered. The results of this research complement the assessment and scoring of the BMS and CF Indexes that ATNi published in 2024. The research will also be considered in updating the methodology for future ATNi Indexes.
Methodology
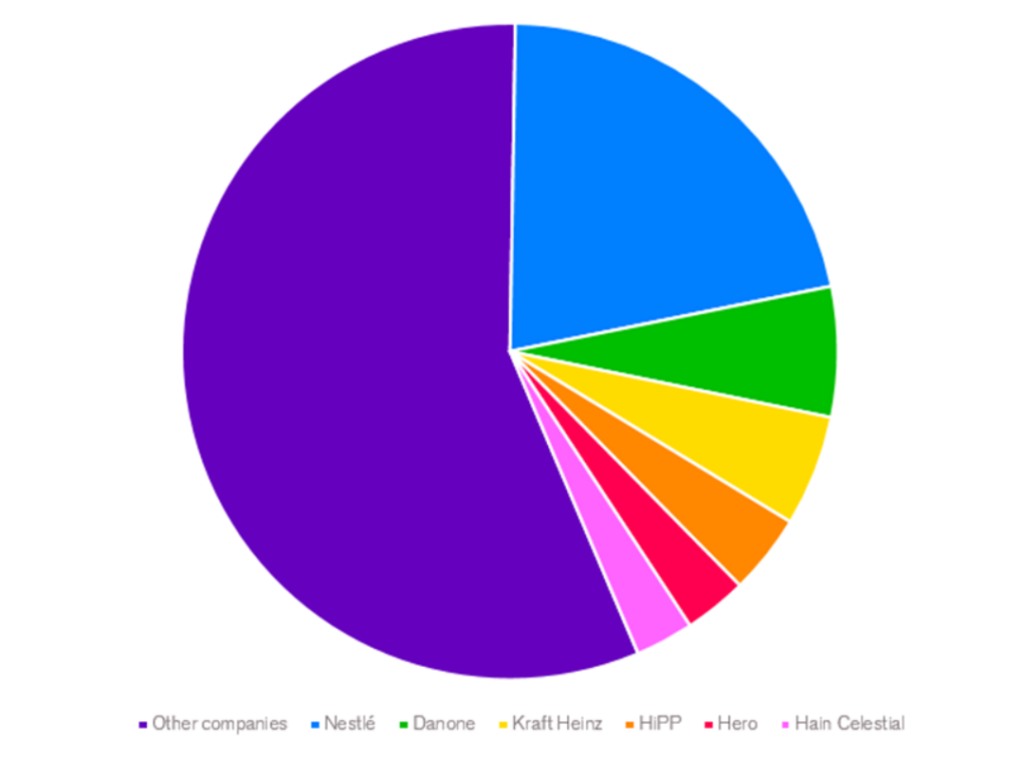
Six companies, who contributed to over 40% of global CACF retail sale estimates in 2021, were selected for the assessment. The countries or markets to include in the research were selected based on the presence of these six companies, where the companies’ CACF shares are high.
To aim for regional representation, at least one country from each of the following WHO regions was selected: the Americas, Eastern Mediterranean, Europe, and South-east Asia. An additional requirement was the availability of data about the companies’ CACF products sold in a country.
Overall Results
Results of the combined assessment of CACF nutrient composition and labelling practices:
None of the companies’ CACF products met all requirements of the NPPM, for both nutrient composition and labelling. Therefore, based on the NPPM none of the CACF are suitable for promotion to infants and young children between six months up to three years of age.
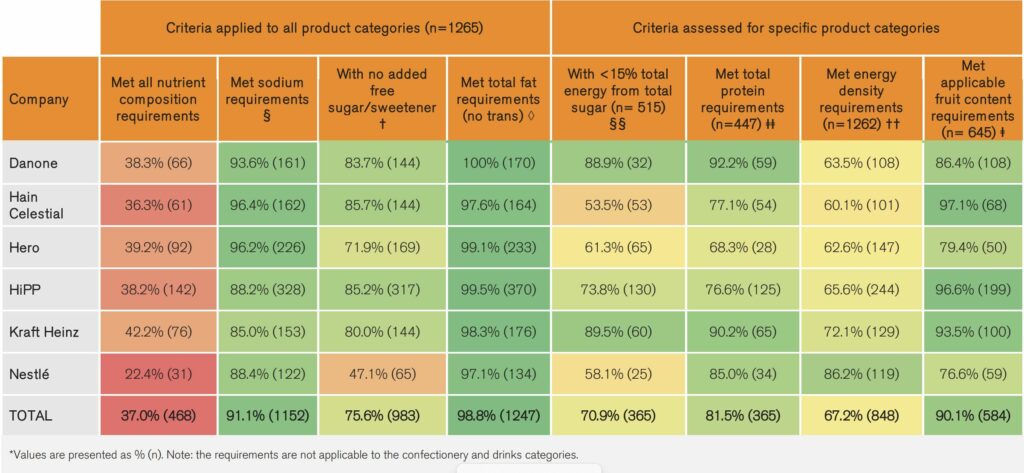
Almost 37% of assessed CACF met all nutrient composition criteria. Most companies’ CACF met the NPPM requirements for fat, sodium, fruit content, and protein. Companies scored the lowest on the requirement related to energy density. Around 67% of relevant CACF had appropriate energy levels to ensure provision of adequate nutrition.
None of the companies’ CACF met the requirements relating to claims, as all products had at least one type of claim (nutritional, health or promotional). None of the puréed CACF met the recommended upper age limit of 12 months either
Recommendations
- Companies selling CACF products are encouraged to adopt the most comprehensive existing guidance on the nutritional composition and labelling of CACF. WHO Europe’s nutrient and promotion profiling model for CACF is the only currently existing and widely recognized model for CACF requirements (see pages 8 and 9 of the NPPM for specific guidance for companies).
- Companies should also continuously strive to improve their CACF products in line with relevant developments in national, regional, and global guidance.
- Companies are urged to improve energy density levels of their CACF to meet NPPM thresholds and follow the WHO recommendation which states “Manufacturers should be encouraged to reduce and declare the water content of products to increase energy density and quality of soft foods and purées. Setting a minimum energy density of 60 kcal /100 g for many purées will also encourage manufacturers to add less water for cooking and blending and will therefore ensure higher quality and better value products.”
- Policymakers should develop new, or update existing, national regulations on the nutrient composition and labelling practices of CACF, in line with international guidance. These 60 regulations should prohibit the use of added sugars and sweeteners, limit sugar and sodium content and outlaw the use of misleading marketing and labelling practices.
- Governments should establish a system for monitoring and evaluating national regulations on CACF.
- Investors are encouraged to make use of existing nutrition frameworks such as ATNI’s Investor Expectations on Nutrition, Diets and Health and to integrate nutrition into responsible investment strategies.
- Investors can use the findings of this report to drive companies’ progress on CACF nutrition through various investment strategies, calling for transparency and adherence to the NPPM.
- Investors can use the data from this report to develop materials to support engagement with companies

