
CF Index 2024
The CF Marketing Index assesses the marketing of commercial complementary foods.
Introduction
This CF Marketing Index 2024 is the first standalone Access to Nutrition Index on companies that produce and distribute CF. It builds on a decade-plus of ATNI experience assessing compliance of companies that sell baby foods with the 1981 International Code of Marketing of Breast-milk Substitutes (‘the Code’). The Code provides guidelines for the responsible marketing of breast-milk substitutes (BMS), including commercial CF for infants and young children. The NetCode protocol/toolkit and the World Health Organization (WHO) Guidance on Ending the Inappropriate Promotion of Foods for Infants and Young Children were used as reference for this research.
ATNI’s research and Indexes do not assess compliance with local regulations or laws, but rather assess private sector performance against international standards and guidance.
The CF Marketing Index 2024 assesses ten of the largest companies that sell CF products, and for which CF amount to at least 5% of their estimated global baby food sales.
Together, these companies are estimated to account for 49% of the global sales of commercial CF products. This Index has two components weighing in equally to a total CF score: a Corporate Profile assessment of the quality of companies’ CF marketing policies, management systems and their level of transparency; and a Country Study assessment of marketing practices in five global markets (China, Germany, Indonesia, Viet Nam, and the US).
Read the full report hereThe CF Marketing Index assesses the marketing of commercial complementary foods, hereinafter referred to as CF products. These include baby porridge and cereals, dairy/fruit/vegetable-based baby purées, savory meals, and snack foods, as well as baby teas, juices, and water for infants and young children aged 6-36 months.
The 10 companies assessed on ATNI’s CF Marketing Index 2024 represent an estimated 49% of the global CF market.
Read the executive summary
Context
The CF Index 2024 assesses companies' CF marketing policies and management systems, their level of transparency, and their marketing practices for the world's largest 10 manufacturers.
Companies are assessed on two main components:
– The Country Studies , which measure companies’ marketing practices in selected countries. For this Index, five countries were selected representing the companies’ primary baby food markets: China, Germany, Indonesia, Viet Nam and the US.
– The Corporate Profile assessment, which examines global corporate policies and procedures and level of disclosure.
The Corporate Profile and Country Studies evaluate the extent to which company policies and practices align with the various provisions of the Code. While the Corporate Profile assesses company policies and commitments on all aspects of the Code, the Country Studies assess marketing practices against specific provisions of the Code.
CF products marketed to infants aged under six months are considered unwanted breast-milk substitutes, as these products interfere with exclusive breastfeeding in the first six months. However, ATNI will assess these products in the CF Marketing Index rather than the BMS Marketing Index, to emphasize that CF products are intended for older infants and young children aged 6-36 months and should not be introduced to infants aged under six months who should be exclusively breastfed.
Results
Below you can see the results of all assessed companies. None of the 10 companies assessed fully align with the Code.
Key Findings
CF companies have a significant opportunity to improve their marketing policies and practices globally.
None of the companies was found to be fully compliant with the Code and guidance on appropriate promotion of foods for infants and young children in policies and practice.
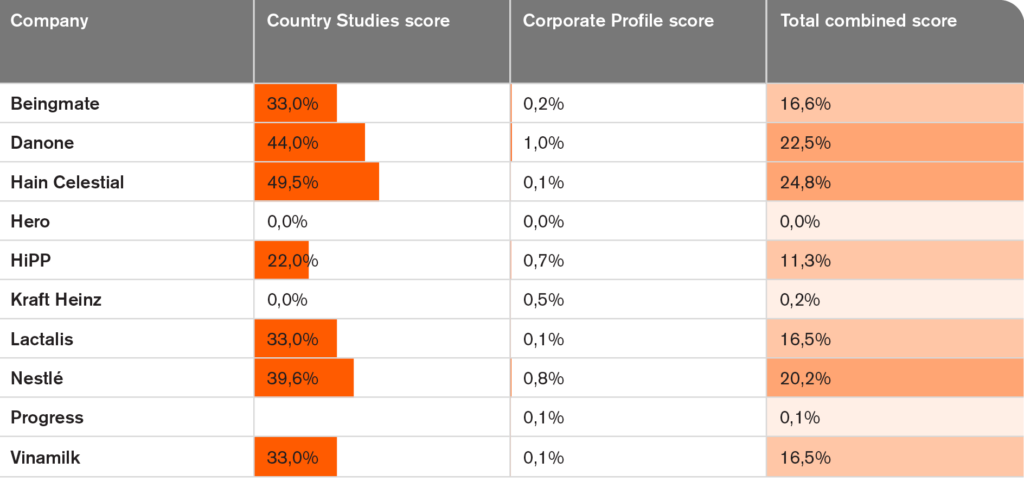
- The highest total CF score is 25% for Hain Celestial. Danone scored 23%, Nestlé 20%, and Beingmate, Lactalis and Vinamilk each scored 17%, while HiPP scored 11%.
- Three companies (Hero, Kraft Heinz and Progress) each scored 0%.
For the Corporate Profile assessment none of the companies had a specific CF marketing policy covering CF products intended for infants 6-36 months. The companies’ scores across nine topics ranged from 0% to 21%. None of the companies showed commitments on ‘marketing messages’ (0%), and ‘avoidance of cross-promotion’ (0%), whereas on ‘guiding principles for Infant and Young Child Feeding’ (21%), ‘implementation and monitoring’ (14%), and ‘lobbying and influencing’ (14%) companies showed more commitments.
Danone, HiPP, Nestlé and Kraft Heinz showed more commitments across the nine topics compared to the other companies. All companies obtained a geographic ‘penalty’ of 90% because no information was found or presented on if and how commitments are upheld in companies’ markets around the world. The average corporate profile score was 3.5%, dropping to 0.4% after the geographic penalty was applied. Danone had the highest score (1%) followed by Nestlé (0.8%), HiPP (0.7%) and Kraft Heinz (0.5%). The other companies had scores less than 0.2%.
Marketing practices were assessed for nine out of the ten. The combined overall score for marketing practices in the five specific markets shows an average score of 30% across all companies, marketing channels and labels assessed. This indicates substantial room for improvement in adherence to the Code and the WHO guidance on ending inappropriate promotion of foods for infants and young children. The highest average compliance across countries was recorded in Viet Nam (44%), followed by Indonesia and China (both at 33%). The lowest levels of compliance were identified in Germany (25%) and in the US (20%).
None of the companies assessed achieved complete compliance with the Code in any of the countries included in this study. Hero and Kraft Heinz both scored low compliance overall (0% on average), indicating a low level of compliance of Hero in Germany and the US, and of Kraft Heinz in China and Indonesia. HiPP, Lactalis, Beingmate and Vinamilk show medium levels of compliance with scores between 22% (HiPP) and 33% (the other three). Hain Celestial, Danone and Nestlé show higher levels of compliance with average scores of 50%, 44% and 40% respectively.
A total of 449 incidences of inappropriate baby food marketing not compliant with the Code and WHO guidance were identified across all companies in the five markets they were assessed in. These incidences were almost evenly distributed across product labels (239) and online channels (205), while only five occurrences were found in traditional media.
A total of 155 incidences of non-compliance with the Code concerned CF products sold by Nestlé, and in descending order 90 incidences were observed for HiPP, 65 for Kraft Heinz, 52 for Hero, 39 for Danone, 14 incidences for Hain Celestial, 12 for Beingmate (all in China), 11 for Lactalis, and 11 for Vinamilk (all in Viet Nam).
Recommendations
To better support our research, we are sharing below recommendations for key stakeholders, focusing on improving the current situation.
Recommendations to CF companies
Companies should prioritize efforts to enhance and harmonize compliance with the Code, specifically the WHO Guidance on Ending the Inappropriate Promotion of Foods for Infants and Young Children supported by WHA Resolution 69.9, across all countries they operate in, to protect optimal nutrition for infants and young children globally.
- Companies are urged to follow WHO guidance relating to complementary feeding and uphold globally recognized public health guidelines, with a key requirement being not to produce and sell commercial CF for infants aged under six months, which directly interferes with exclusive breastfeeding up to six months.
- Companies are urged to especially follow recommendations 3, 4, 5, and 6 of the WHA 69.9 supported guidance, to ensure the appropriate promotion of their CF products across all marketing channels.
- To comply with the recommendations of the WHA 69.9 supported guidance, companies should ensure that CF products are of appropriate nutritional quality in line with the latest WHO guidelines, such as the NPPM.
- Based on the results of the country studies, companies are especially encouraged to state the appropriate recommended age of introduction (this should not be under six months), include messages that protect breastfeeding and appropriate complementary feeding practices, and refrain from using claims when marketing CF products. Companies should also refrain from cross-promotion, whereby BMS are indirectly promoted through the promotion of CF.
- Based on ATNI’s findings, companies should have explicit commitments and guidelines on responsibly marketing their CF products in digital environments, in line with the latest digital marketing guidance by WHO. Recommendation 4 of the digital marketing guidance specifically recommends restricting in digital environments the promotion of CF that do not meet relevant nutrition standards and that are marketed as suitable for infants less than six months of age.
- In addition, the appropriate promotion of CF in digital environments requires that the marketing messages criteria outlined in the WHO Guidance on Ending the Inappropriate Promotion of Foods for Infants and Young Children are followed, to ensure that messages supporting optimal IYCF are present and that inappropriate messages which could undermine recommended practices are not included.
- Considering the above recommendations, companies are urged to develop and/or publish policies dedicated to the responsible marketing of CF that fully align with the Code, specifically the WHO Guidance on Ending the Inappropriate Promotion of Foods for Infants and Young Children supported by WHA Resolution 69.9.
- Companies should bolster their management systems to deliver consistent compliance with their stated commitments, once brought into full alignment with the Code.
- Companies should promptly take corrective actions upon receiving reports of incidences of non-compliance with the Code.
- ATNI urges companies selling CF to take responsibility for monitoring their marketing practices beyond local regulations, according to the principles and the aim of the Code and subsequent relevant resolutions, namely WHA Resolution 69.9.
- Companies should publish more information on their CF marketing policies and practices to increase transparency, and make key documents easily accessible to stakeholders.
Recommendations to investors
As shareholders, investors play a significant role in shaping food companies’ governance, strategy, and disclosure practices.
- Investors should make use of existing models, such as WHO’s designated model to assess complementary foods (NPPM), to integrate compliance with WHO guidelines on the appropriate promotion of CF products into responsible investment strategies.
- Investors can use the findings of this Index to drive companies’ progress on responsible CF marketing practices through various investment strategies, calling for transparency and compliance to the Code, specifically the WHO Guidance on Ending the Inappropriate Promotion of Foods for Infants and Young Children supported by WHA Resolution 69.9.
- Investors can use the data from this report to develop materials to support engagement with companies.
- Investors are urged to employ their influence to encourage companies to take steps towards full Code compliance.
Recommendations to policymakers
- Following WHO guidance supported by WHA Resolution 69.9, governments should put in place measures to end the inappropriate promotion of food for infants and young children and promote enabling environments that support parents and caregivers to make well-informed feeding decisions.
- Governments can make use of existing resources, such as the Compendium of international standards and guidelines for the improved composition and labelling of commercially produced complementary foods in Southeast Asia, developed by UNICEF East Asia and the Pacific Regional Office for the COMMIT Initiative, which helps guide the development of new, or update existing, national regulations on the nutrient composition and labeling practices of commercial CF, in line with international guidance
- We suggest that particular focus be placed on restricting parallel imports to countries and recommend consideration of stricter rules to prevent the entry and marketing of parallel import products that do not comply with national regulations. The authorities could, perhaps, look at how the importation of these products might be more strictly controlled.
Recommendations to civil society and non-governmental organizations (NGOs)
- Educational campaigns targeting consumers should be conducted to raise awareness on appropriate complementary feeding practices, and the potential risks associated with the inappropriate marketing of CF products.
- NGOs, civil society, and academia can contribute to expanding the research and body of evidence on the marketing of CF, to support the development of comprehensive guidelines that address concerns over the marketing of these product.
Companies Assessed
Click on the tiles below for a detailed overview of individual companies' performance and key recommendations.

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard

Go to company scorecard
Methodology
This Index includes a Corporate Profile and country assessments, which together assess companies’ nutrition policies and practices, globally and in the selected markets.
The increased number of selected companies and countries assessed for the BMS and CF Marketing Indexes 2024, as well as the separate assessments of BMS and CF marketing, warranted several adjustments to the methodology. ATNI undertook an extensive consultation process in which these changes were discussed.
The methodology for the BMS and CF Marketing Indexes 2024 includes additional information about the companies selected and the respective Index(es) they are assessed in, as well as the basis and nature of the assessments for each component and how they feed into the overall Indexes.
Read the full methodology here
Acknowledgements & Disclaimers
This report is being supported by the Bill & Melinda Gates Foundation. The writing of this report, the underlying methodology development and the research were conducted by the Access to Nutrition
Initiative Infant and Young Child Nutrition project team, which consists of Efi Chatzinikolaou, Lucy Cosenza, Daniela Hernández Morales, Ludovica Ibba, Nadine Nasser, Marina Plyta, Irene Santoro, and Mark Wijne. We would also like to thank colleagues for their support in various steps of the process: Babs Ates, Freddie von Kaufmann, Eaindra Aye, Aurélie Reynier, Omari Palmer, Vrinda Poojari, and Philip Eisenhart as well as Juliana Constantino, who supported the research as part of her internship at ATNI.
The ATNI team drew on the expertise and advice of the ATNI BMS expert group members Elizabeth Zehner, Laurence Grummer-Strawn (observer), Linda Meyers, Shelly Sundberg, and Shiriki Kumanyika and would like to thank them for their valuable input throughout this research and the underlying methodology. The views expressed in this report, however, do not necessarily reflect the views of the group’s members or their institution.
Underlying data for the country studies has been sourced from Digimind, Innova Market Insights, and Nielsen Ad Intel International under license.
ATNI would like to thank Kummer & Herman and Studio September for design, Wren Media for editing and proofreading, 73Bit for setting up the data platform and M&C Saatchi for communications.

